Alzheimer’s Disease: The most common form of dementia
An irreversible, progressive neurologic disorder that slowly degrades memory, thinking and social skills that affects a person’s ability to function independently.
In October 2022, we commenced a Phase2b of Trappsol® Cyclo™ for the treatment of early Alzheimer’s Disease (AD), targeting the reduction of amyloid beta and tau.
Affects more than 5 million people in the U.S.1
6th leading cause of death in the U.S.1
500,000 new cases every year2
8 million cases projected by 20501

Trappsol® Cyclo™ is a proprietary formulation of hydroxypropyl beta cyclodextrin and in multiple clinical studies has shown encouraging results to effectively manage the transportation of cholesterol. Many of the known risk factors for Alzheimer’s are associated with cholesterol metabolism. Cholesterol imbalance in Alzheimer’s patients is well known, and significant research exists suggesting these imbalances are responsible for amyloid beta (Aβ) and tau accumulation. Furthermore, neurons, because of their high metabolic demands, experience an increased level of oxidative stress. Oxidative stress has also been linked to abnormal cholesterol accumulation and processing. Alzheimer’s shares features with NPC-1, a neurovisceral, genetic disease in which cholesterol accumulates in lysosomes, including progressive decline in cognitive ability, amyloid beta plaques in the CNS, and increased levels of tau in the cerebrospinal fluid (CSF).
Alzheimer’s Disease has Similarities with Niemann-Pick Disease Type C
-
-
Cognitive decline
-
Elevated levels of tau
-
Amyloid plaques
-
Trappsol® Cyclo™ for the Treatment of Alzheimer’s Disease
Targeting Reduction of Amyloid Beta and Tau
Alzheimer’s Mini-Mental State Evaluation Performance1
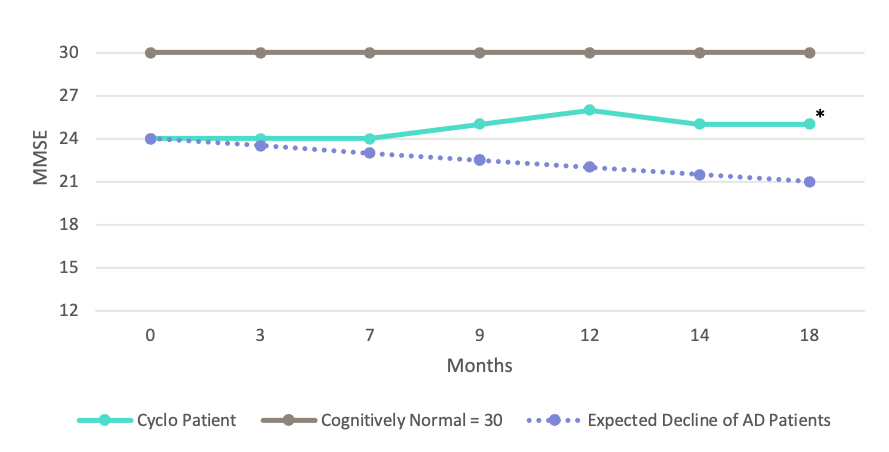
Encouraging Results in Alzheimer’s Patient Under Compassionate Use Program
FDA authorized use of Trappsol® Cyclo™ in geriatric patient
18 months of monthly IV infusion
Disease did not progress
Family reported less volatility and greater word finding ability
*Treating physician reported the 18-month score as a range between 24-26
1: Rate of MMSE decline in AD patients: Eldholm, RS et al, J. Alz. Disease, 61: 1221, 2018. Suh, GH et al., Intl. J. Geriatric Psychiatry, 19(9): 817, 2004.
Trappsol® Cyclo™ is not currently approved for any indication.
Third-party websites are provided for convenience only. Cyclo Therapeutics, Inc. does not approve of, or endorse any of the content. Cyclo Therapeutics, Inc. does not maintain, control or monitor the content of third-party websites in any way.
